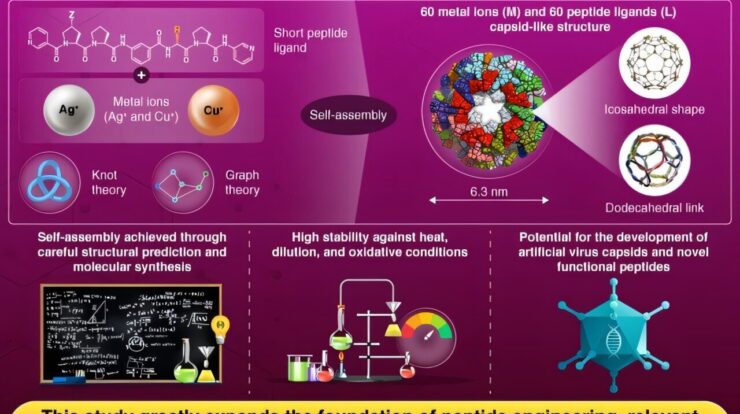
Managing the layout and configuration of intertwined molecular chains is a significant hurdle in molecular engineering, especially when aiming to build sizable nanostructures that resemble biological entities. Natural instances like viral shells and transport proteins highlight the impressive capabilities of these designs. Nonetheless, techniques for fabricating extensive, hollow nanoforms with exact geometrical precision have been hard to come by until recently.
A research group headed by Associate Professor Tomohisa Sawada at the Institute of Science Tokyo, Japan, has managed to create a molecular spherical shell configuration featuring the geometric topology of a regular dodecahedron.
This pioneering study, released online in the journal
Chem
explains how the scientists constructed this sizable formation, which has an external diameter of 6.3 nanometers, by intertwining peptides with metallic ions.
Sawada elucidates that the creation of this intricate framework was guided by geometrical principles and forecasts, which culminated in introducing a novel idea: controlling chemical configurations through geometry.
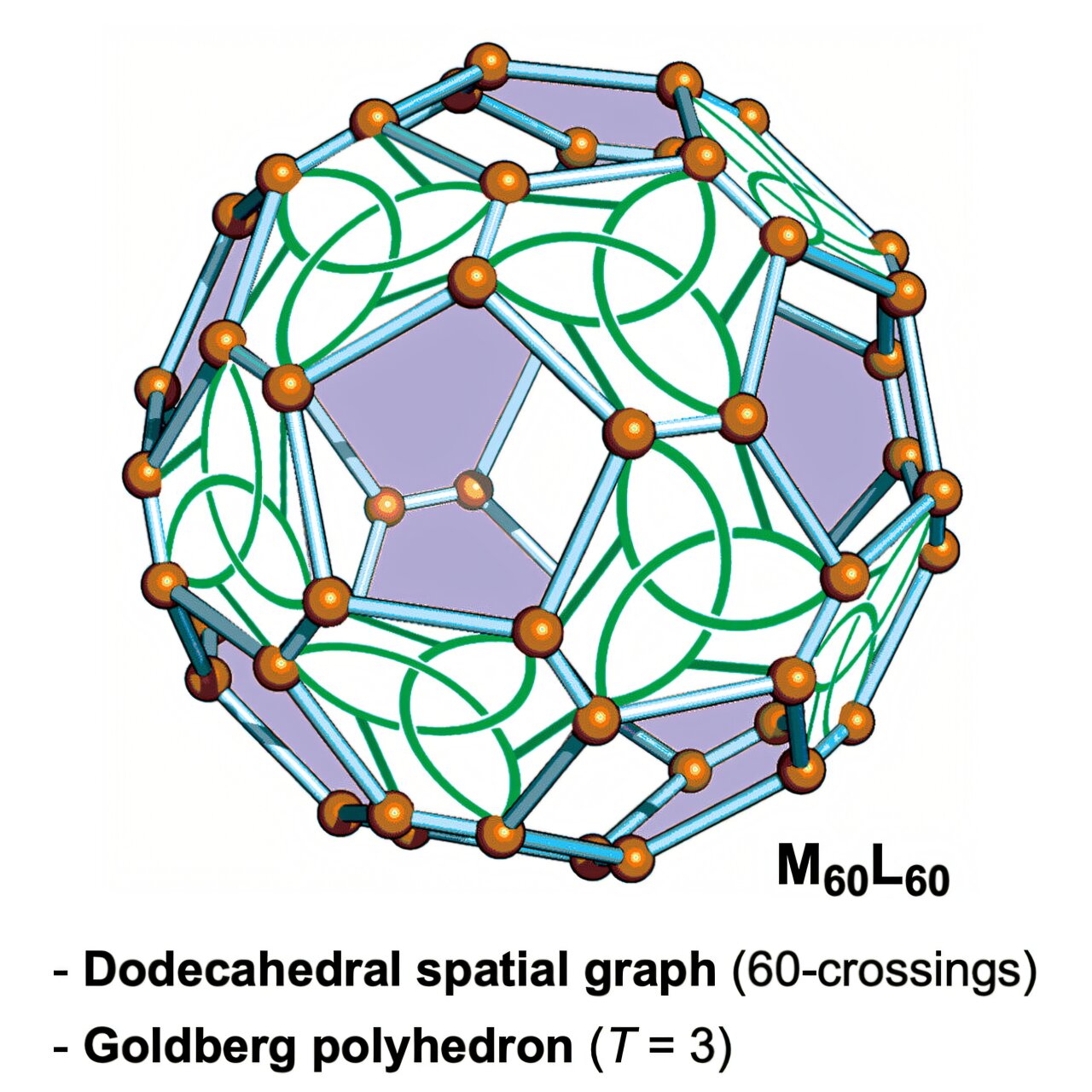
The team’s strategy merged two different mathematical approaches—knot theory and graph theory—to forecast and subsequently realize the self-assembly of a never-before-seen dodecahedral link featuring 60 crossings, made up of 60 metal ions paired with 60 peptide ligands.
60
L
60
).
Previously, the researchers had constructed smaller structures using tetrahedral and cubic connections. Nonetheless, a more intricate dodecahedral connection arose when they made additional alterations to the peptide sequence while trying to functionalize it.
24
L
24
a more diminutive cubic link.
X-ray crystallography showed that the resultant Mصند
60
L
60
The metal-peptide shell encompasses an internal cavity roughly 4.0 nanometers in size (about 34,000 Å).
3
) that is sufficiently spacious to contain macromolecules like proteins or nanomaterials.
Apart from its remarkable structural intricacy, the Mصند
60
L
60
The shell demonstrated significant resilience under thermal stress, when diluted, and in oxidative environments, attributes that the scientists linked to its distinctive interwoven structural framework.
The group further showed that the exterior of the capsid could be altered with different functional groups without compromising its structure, paving ways for tailored modifications according to particular requirements.
These features make M
60
L
60
a promising platform for different uses, such as drug delivery systems and molecular transport.
“Sawada emphasizes that due to the variety and adaptability of peptide structures, our approach has significant advantages over DNA origami technology when it comes to adding functionality to these structures,” he notes.
Furthermore, as our method includes theoretical predictions along with trial-and-error experiments, we occasionally achieve remarkable structures that surpass our expectations—a true testament to the nature of chemistry.
In total, this study marks a considerable advancement in comprehending the development of artificial virus capsid-like structures.
“Our discoveries substantially broaden the basis of peptide engineering and are expected to have profound impacts across multiple areas such as molecular self-assembly, materials chemistry, and mathematical theories,” states Sawada.
The researchers are now setting their sights on even more ambitious structures, imagining Mصند
180
L
180
and M
240
L
240
Assemblies with 180 and 240 crossings, respectively, as their upcoming challenges.
More information:
Yuuki Inomata and colleagues presented an M60L60 metal-peptide capsule featuring a 60-crossing interwoven structure.
Chem
(2025).
DOI: 10.1016/j.chempr.2025.102555
Furnished by the Institute of Science Tokyo
This story was originally published on
Dailyexe
. Subscribe to our
newsletter
for the latest sci-tech news updates.





